Long-term prevention
Given the opportunity to remain in the trial for up to an additional 24 months of ARCALYST treatment, 99% of eligible participants chose to continue treatment in the long-term extension.
While on treatment in the long-term extension, arcalyst continued to deliver powerful results:

Reduction in risk of flares in those who continued treatment vs those who suspended treatment
In participants who continued in the long-term extension more than 18 months after their last flare:
- 3% (1 of 33) of those who continued taking ARCALYST had a flare. This occurred during a temporary interruption (4 weeks) in ARCALYST treatment
- 75% (6 of 8) who suspended ARCALYST treatment for observation had a flare following discontinuation
These results are consistent with the 96% risk reduction seen above.
ARCALYST may be used for the duration of your disease
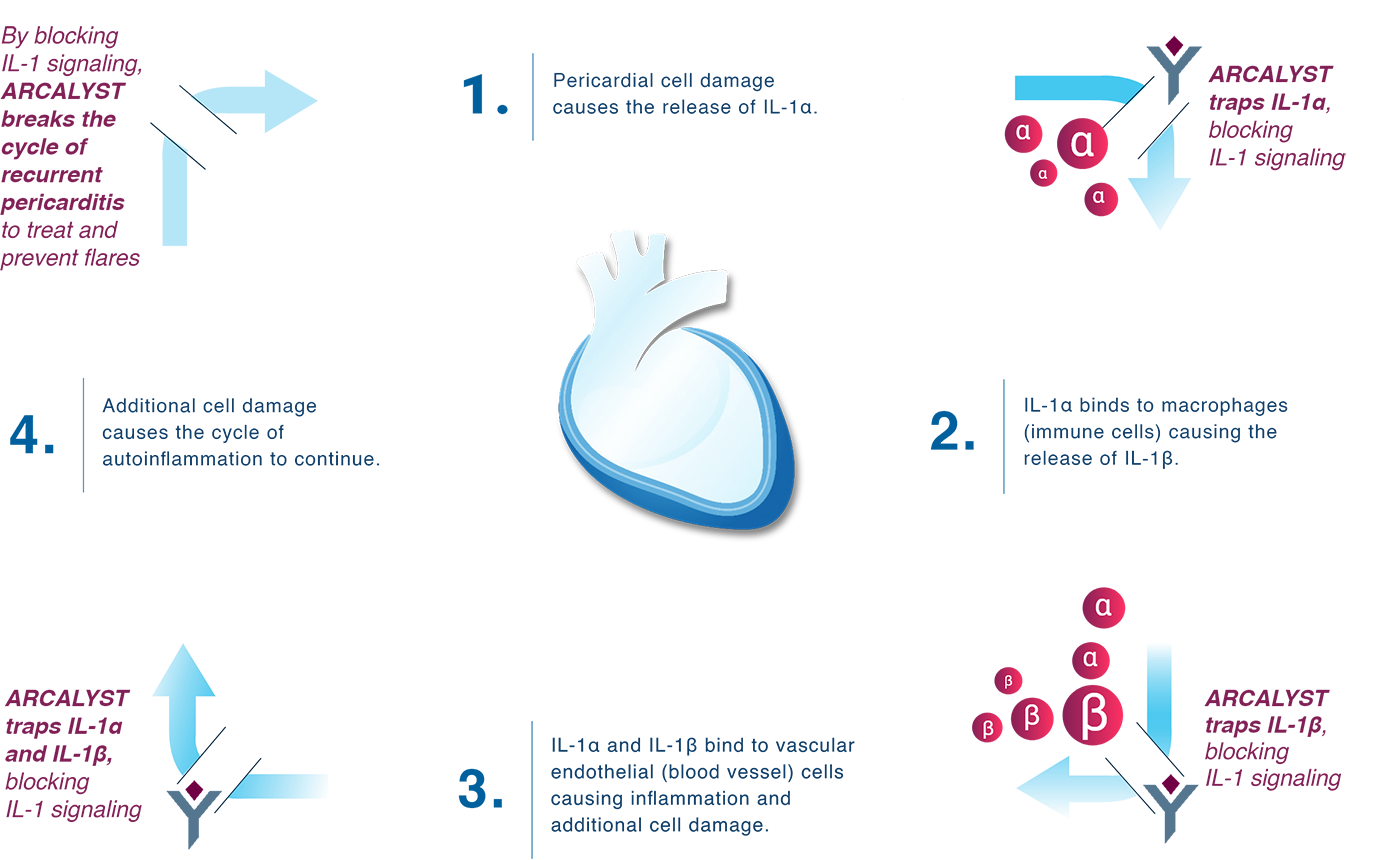
Recurrent pericarditis is an unpredictable disease. For some people it can last months. For others it can last years. While certain treatments used for first or one-time episodes may not be appropriate for long-term use, ARCALYST may be used until the cycle of autoinflammation has resolved.
ARCALYST was studied in people diagnosed with recurrent pericarditis who were experiencing at least a second recurrence
First Period
Reduced pain and inflammation and time to transition to ARCALYST alone were evaluated in 86 participants taking ARCALYST.
Second Period
Risk of recurrence and days with minimal or no pain were subsequently evaluated in 61 participants, 30 who continued taking ARCALYST compared with 31 of whom were switched to placebo.
Long-term extension Period
74 of 75 eligible participants chose to continue treatment in the long-term extension of up to 2 years. 52 remained on treatment for 18 months after their last flare. Of these participants, 33 continued treatment with open-label ARCALYST, 8 suspended treatment and remained in the trial for observation, and 11 exited the trial.
safety
What is the most important information I should know about ARCALYST?
ARCALYST can affect your immune system. ARCALYST can lower the ability of your immune system to fight infections. Serious infections, including life-threatening infections and death, have happened in people taking ARCALYST. Taking ARCALYST can make you more likely to get infections, including life-threatening serious infections, or may make any infection that you have worse.
You should not begin treatment with ARCALYST if you have an infection or have infections that keep coming back (chronic infection).
After starting ARCALYST, if you get an infection, any sign of an infection, including a fever, cough, flu-like symptoms, or have any open sores on your body, call the healthcare provider right away. Treatment with ARCALYST should be stopped if you get a serious infection.
You should not take medicines that block tumor necrosis factor (TNF), such as Enbrel® (etanercept), Humira® (adalimumab), or Remicade® (infliximab), while you are taking ARCALYST. You should also not take other medicines that block interleukin-1 (IL-1), such as Kineret® (anakinra), while taking ARCALYST. Taking ARCALYST with any of these medicines may increase your’s risk of getting a serious infection.
Before starting treatment with ARCALYST, tell the healthcare provider if you:
- Think you have an infection
- Are being treated for an infection
- Have signs of an infection, such as fever, cough, or flu-like symptoms
- Have any open sores on your body
- Have a history of infections that keep coming back
- Have asthma. People with asthma may have an increased risk of infection
- Have diabetes or an immune system problem. People with these problems have a higher chance for infections
- Have tuberculosis, or if you have been in close contact with someone who has had tuberculosis
- Have or have had HIV, hepatitis B, or hepatitis C
- Take other medicines that affect your immune system
Before you begin treatment with ARCALYST, talk with the healthcare provider about your vaccine history. Ask the healthcare provider whether you should receive any vaccines, including the pneumonia vaccine and flu vaccine, before you begin treatment with ARCALYST.
What are the possible side effects of ARCALYST?

What are the possible side effects of ARCALYST?
ARCALYST can cause serious side effects, including:
Serious side effects:
- Serious Infections. ARCALYST can affect your immune system. ARCALYST can lower the ability of your immune system to fight infections. Serious infections, including life-threatening infections and death, have happened in people taking ARCALYST. Taking ARCALYST can make you more likely to get infections, including life-threatening serious infections, or may make any infection that you or your child have worse.
- Risk of Cancer. Medicines that affect the immune system may increase the risk of getting cancer.
- Allergic Reaction. Stop taking or giving ARCALYST and call the healthcare provider or get emergency care right away if you get any of the following symptoms of an allergic reaction while taking ARCALYST:
- Rash
- Swollen face
- Trouble breathing
- Changes in your blood cholesterol and triglycerides (lipids). Your healthcare provider will do blood tests to check for these changes.
Common side effects:
- In people with CAPS and RP, the most common side effects of ARCALYST include:
- Injection-site reactions including: pain, redness, swelling, itching, bruising, lumps, inflammation, skin rash, blisters, warmth, and bleeding at the injection site.
- Upper respiratory tract infections
- Joint and muscle aches in RP
- In people with DIRA, the most common side effects of ARCALYST include:
- Upper respiratory tract infections
- Rash
- Ear infection
- Sore throat
- Runny nose
These are not all the possible side effects of ARCALYST. Tell your healthcare provider if you have any side effect that bothers you or does not go away. For more information, ask your healthcare provider or pharmacist.
CAPS, cryopyrin-associated periodic syndromes; DIRA, deficiency of interleukin-1 receptor antagonist.






